Many concrete structures suffer from corrosion of reinforcing steel due to chloride penetration and carbonation, and it is common for both deteriorations to progress simultaneously. Carbonation of concrete significantly changes the properties of concrete, so that cement hydrates is decomposed and the microstructure is also changed. The chloride penetration of carbonated concrete is bound to be significantly different compared to non-carbonated concrete. For this reason, chloride penetration parameters of carbonated cementitious materials were examined in this paper, i.e., (a) surface chloride content, (b) chloride diffusivity, (c) chloride adsorption capacity, and (d) critical chloride content. Each material parameter was calculated from the material parameter model reflecting the change in porosity due to carbonation. Carbonation of concrete converts cement hydrates into Calcite, resulting in almost loss of chloride adsorption capacity. Even though the pH is slightly decreased due to carbonation, critical chloride content of carbonated cementitious materials was calculated, confirming that the reinforcement is very vulnerable to corrosion. Since the main parameters are affected by mixing properties of cementitious materials, the analysis was performed for concrete mixes with arbitrary assumed conditions. Since the parameters change at each cement hydration stage, their time evolution was expressed. This study is expected to be useful to develop a chloride penetration model of carbonated cementitious materials in the future.
| Published in | Journal of Civil, Construction and Environmental Engineering (Volume 10, Issue 3) |
| DOI | 10.11648/j.jccee.20251003.13 |
| Page(s) | 123-130 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2025. Published by Science Publishing Group |
Carbonation, Chloride Transport, Carbonation, Cement Hydration, Microstructure
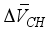 + [C-S-H]
+ [C-S-H] 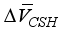 (1)
(1) 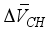 and
and 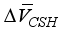 are equivalent to 3.85 × 10-6 m3/mol, 15.39 × 10-6 m3/mol, respectively. The above formula is the reduced the amount of pore in carbonated concrete, and the reduced amount of pore (cp) for cement paste can be expressed as follows considering volumetric fraction of the cement paste, Vcp, in unit volume concrete.
are equivalent to 3.85 × 10-6 m3/mol, 15.39 × 10-6 m3/mol, respectively. The above formula is the reduced the amount of pore in carbonated concrete, and the reduced amount of pore (cp) for cement paste can be expressed as follows considering volumetric fraction of the cement paste, Vcp, in unit volume concrete. 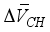 + [C-S-H]
+ [C-S-H] 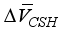 }(2)
}(2) 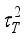 ), (d) hindrance effect due to narrow pore walls, F(H), and so on. In what follows a description of these factors is given
), (d) hindrance effect due to narrow pore walls, F(H), and so on. In what follows a description of these factors is given 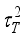 ) ·F(H)(9)
) ·F(H)(9) 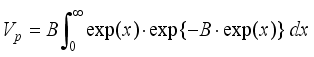 (11)
(11) 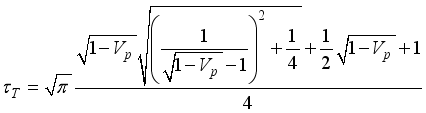 (12)
(12) 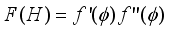 (13)
(13) 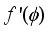 and
and 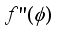 , are related to the reduced pore diameter
, are related to the reduced pore diameter 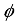 .
. 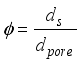 (14)
(14) 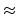 3.34×10-8 cm for CO2 gas), dpore: diameter of pore.
3.34×10-8 cm for CO2 gas), dpore: diameter of pore. 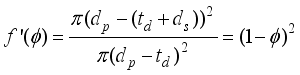 (15)
(15) 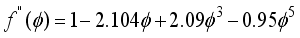 (16)
(16) 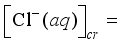
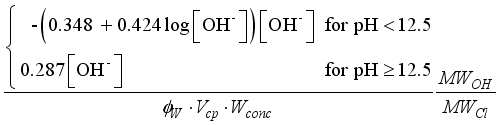 (19)
(19) 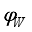 : volumetric fraction of water in pore system.
: volumetric fraction of water in pore system. | [1] | Page, C. L., and Page, M. M. Durability of Concrete and Cement Composite, Woodhead Publishing in Materials, Oxford, U.K, 2007. |
| [2] | Maekawa, K., Ishida, T., and Kishi, T. Multi-scale Modelling of Structural Concrete, Taylor & Francis, 2009, 243-244, |
| [3] | JSCE, Concrete Standard Specification, Part of durability, 2001. |
| [4] | Bermudez, M. A., and Alaejos, P. Models for Chloride Diffusion Coefficients of Concrete in Tidal Zone. ACI Materials Journal, 2010, 107(1), 3-11. |
| [5] | Wan, Z. M., Wittmann, F. H., and Zhao, T. J., and Fan, H. Chloride Content and pH Value in the Pore Solution of Concrete under Carbonation, Journal of Zhejiang University-SCIENCE (Applied Physics & Engineering), 2013, 14(1), 71-78, |
| [6] | CEB. Durable Concrete Structures: Design Guide, 2nd Edition, Thomas Telford, London, 1992. |
| [7] | Kobayashi, K., Siraki, R., and Kawai, K. Migration and Concentration of Chlorides, Sulphates, and Alkali Compounds in Concrete Caused by Carbonation (written in Japanese), Concrete Research and Technology, 1990, 1(2), 69-82. |
| [8] | Geng, J., Easterbrook, D., Liu, Q., and Li, L. Effect of carbonation on release of bound chlorides in chloride contaminated concrete, Magazine of concrete research, 2015, 1-11, |
| [9] | Yoon, I. S., Copuroglue, O., and Park, K. Effect of Global Climatic Change on Carbonation Progress of Concrete, Atmospheric Environment, 2007, 41, 7274-7285, |
| [10] | Ngala, V. T., and Page C. L. Effect of Carbonation on Pore Structure and Diffusion Properties of Hydrated Cement Pastes, Cement and Concrete Research, 1997, 27(7), 995-1007. |
| [11] | Dias, W. P. S. Reduction of Concrete Sorptivity with Age through Carbonation,” Cement and Concrete Research, 2000, 30, 1255-1261. |
| [12] | Papadakis, V. G., and Vayenas, C. G. Physical and Chemical Characteristics Affecting the Durability of Concrete, ACI Materials Journal, 1991, 8(2), 186-196. |
| [13] | Yoon, I. S., and Chang, C. Quantitative Relationship between Chloride Penetration Depth and Hydraulic Conductivity of Concrete under Hydrostatic Pressure, Journal of Material Engineering, ASCE. 2022, |
| [14] | Van Breugel, K. (1991). Simulation of hydration and formation of structures in hardening cement-based materials, Ph. D Dissertation, TU Delft, The Netherlands. |
| [15] | Kassir, M. K., and Ghosn, M. (2002). Chloride-induced Corrosion of Reinforced concrete Bridge Decks, Cement and Concrete Research, 32(1), 139-143, |
| [16] | Yoon, I. S. Comprehensive Approach to Calculate Oxygen Diffusivity of Cementitious Materials Considering Carbonation, International Journal of Concrete Structures and Materials, 21, 2018, |
| [17] | Wilke, C. R. and Chang, P. Correlation of Diffusion Coefficient in Dilute Solutions, Journal of American Institute of Chemical Engineering, 1, 1995, |
| [18] | Welty, J. R., Wicks, C. E., Wilson, R. E., and Rorrer, G. Fundamentals of Momentum, Heat, and Mass Transfer, 4th Edition, John Wiley & Sons, 2001. |
| [19] | Yoon, I. S., Shin, H., and Thomas, K. Comparative Study on Performance of Corrosion Protective Systems for Posttensioned Concrete Members,” ACI Structural Journal, 2019, 116(3), 273-284. |
| [20] | Yoon, I. S., Saeki, T., and Park, S. Chloride Ions Binding Behavior of Cement Hydrates, RILEM Proceedings PRO83: Microstructural Related Durability of Cementitious Composites, 2012. |
| [21] | Yoon, I. S., and Saeki, T. Mechanism of Chloride Adsorption in Cement Hydrates as per the Time Dependent Behavior, ACI Materials Journal, 2021, 118(1), 1-13, |
| [22] | Gouda, V. K. Corrosion and Corrosion Inhibition of Reinforcing Steel. British Corrosion Journal, 1970, 5, 198-203. |
| [23] | Hausmann, D. A. Steel Corrosion in Concrete. How Does It Occur, Journal of Materials Protection, 1996, 5, 19-23. |
APA Style
Yoon, I. (2025). Chloride Transport Parameters of Carbonated Concrete. Journal of Civil, Construction and Environmental Engineering, 10(3), 123-130. https://doi.org/10.11648/j.jccee.20251003.13
ACS Style
Yoon, I. Chloride Transport Parameters of Carbonated Concrete. J. Civ. Constr. Environ. Eng. 2025, 10(3), 123-130. doi: 10.11648/j.jccee.20251003.13
@article{10.11648/j.jccee.20251003.13,
author = {In-Seok Yoon},
title = {Chloride Transport Parameters of Carbonated Concrete
},
journal = {Journal of Civil, Construction and Environmental Engineering},
volume = {10},
number = {3},
pages = {123-130},
doi = {10.11648/j.jccee.20251003.13},
url = {https://doi.org/10.11648/j.jccee.20251003.13},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.jccee.20251003.13},
abstract = {Many concrete structures suffer from corrosion of reinforcing steel due to chloride penetration and carbonation, and it is common for both deteriorations to progress simultaneously. Carbonation of concrete significantly changes the properties of concrete, so that cement hydrates is decomposed and the microstructure is also changed. The chloride penetration of carbonated concrete is bound to be significantly different compared to non-carbonated concrete. For this reason, chloride penetration parameters of carbonated cementitious materials were examined in this paper, i.e., (a) surface chloride content, (b) chloride diffusivity, (c) chloride adsorption capacity, and (d) critical chloride content. Each material parameter was calculated from the material parameter model reflecting the change in porosity due to carbonation. Carbonation of concrete converts cement hydrates into Calcite, resulting in almost loss of chloride adsorption capacity. Even though the pH is slightly decreased due to carbonation, critical chloride content of carbonated cementitious materials was calculated, confirming that the reinforcement is very vulnerable to corrosion. Since the main parameters are affected by mixing properties of cementitious materials, the analysis was performed for concrete mixes with arbitrary assumed conditions. Since the parameters change at each cement hydration stage, their time evolution was expressed. This study is expected to be useful to develop a chloride penetration model of carbonated cementitious materials in the future.
},
year = {2025}
}
TY - JOUR T1 - Chloride Transport Parameters of Carbonated Concrete AU - In-Seok Yoon Y1 - 2025/06/22 PY - 2025 N1 - https://doi.org/10.11648/j.jccee.20251003.13 DO - 10.11648/j.jccee.20251003.13 T2 - Journal of Civil, Construction and Environmental Engineering JF - Journal of Civil, Construction and Environmental Engineering JO - Journal of Civil, Construction and Environmental Engineering SP - 123 EP - 130 PB - Science Publishing Group SN - 2637-3890 UR - https://doi.org/10.11648/j.jccee.20251003.13 AB - Many concrete structures suffer from corrosion of reinforcing steel due to chloride penetration and carbonation, and it is common for both deteriorations to progress simultaneously. Carbonation of concrete significantly changes the properties of concrete, so that cement hydrates is decomposed and the microstructure is also changed. The chloride penetration of carbonated concrete is bound to be significantly different compared to non-carbonated concrete. For this reason, chloride penetration parameters of carbonated cementitious materials were examined in this paper, i.e., (a) surface chloride content, (b) chloride diffusivity, (c) chloride adsorption capacity, and (d) critical chloride content. Each material parameter was calculated from the material parameter model reflecting the change in porosity due to carbonation. Carbonation of concrete converts cement hydrates into Calcite, resulting in almost loss of chloride adsorption capacity. Even though the pH is slightly decreased due to carbonation, critical chloride content of carbonated cementitious materials was calculated, confirming that the reinforcement is very vulnerable to corrosion. Since the main parameters are affected by mixing properties of cementitious materials, the analysis was performed for concrete mixes with arbitrary assumed conditions. Since the parameters change at each cement hydration stage, their time evolution was expressed. This study is expected to be useful to develop a chloride penetration model of carbonated cementitious materials in the future. VL - 10 IS - 3 ER -